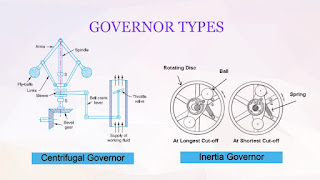
:max_bytes(150000):strip_icc():format(webp)/NalinratanaPhiyanalinmat-EyeEm-5bda1cbbc9e77c0026c7a159.jpg)
The zeroth law of thermodynamics states that if two
systems are both in thermal equilibrium with a third system, then the first two
systems are also in thermal equilibrium with each other.
Key Takeaways: Zeroth Law of
Thermodynamics
- The zeroth law of thermodynamics is one of the
four laws of thermodynamics, which states that if two systems are in
thermal equilibrium with a third system, then they are in thermal
equilibrium with one another.
- Thermodynamics is the study of the relationship between
heat, temperature, work, and energy.
- Most
generally, equilibrium refers to a
balanced state that does not change overall with
time.
- Thermal
equilibrium refers
to the situation where two objects that can transfer heat to each other
stay at a constant temperature over time.
Understanding Thermodynamics
Thermodynamics is the study of the
relationship between heat, temperature, work—which
is performed when a force applied to an object causes
that object to move—and energy, which comes in many forms and is
defined as the capacity to do work. The
four laws of
thermodynamics describe how
the fundamental physical quantities of temperature, energy, and entropy change
in various situations.
As
an example of thermodynamics in action, placing a pot of water on a heated
stove will cause the pot to heat up because heat is transferred to the pot from the stove. This in turn
causes the molecules of water to bounce around in the pot. The faster movement
of these molecules is observed as hotter water.
If
the stove had not been hot, it would not have transferred any thermal energy to
the pot; thus, the water molecules could not have begun moving faster and the
pot of water would not have heated up.
Thermodynamics
emerged in the 19th century, when scientists were building and
improving steam engines, which use steam to help move an object such as a
train.
Understanding Equilibrium
Most
generally, equilibrium refers to a
balanced state that does not change overall with
time. This does not mean that nothing is happening; rather, that two influences
or forces are balancing each other out.
Consider,
for example, a weight hanging from a string attached to the ceiling. At first,
the two are in equilibrium with one another and the string does not break. If
more weight is attached to the string, however, the string will be tugged
downward and may eventually break as the two are no longer in equilibrium.
Thermal Equilibrium
Thermal
equilibrium refers
to the situation where two objects that can transfer heat to each other stay at
a constant temperature over time. Heat can be transferred several ways,
including if the objects are in contact with one another or if heat is radiated
from a source like a lamp or a sun. Two objects are not in thermal equilibrium
if the overall temperature changes with time, but they can approach thermal
equilibrium as the hotter object transfers heat to the colder one.
Consider,
for example, a colder object touching a hotter object—like ice that has been
dropped in a hot cup of coffee. After some time, the ice (later water) and the
coffee will reach a certain temperature that is in between that of the ice and
the coffee. Though the two objects were not in thermal equilibrium at the
beginning, they approach—and eventually
reach—thermal equilibrium, the temperature in between the hot and cold
temperatures.
What Is the Zeroth Law of
Thermodynamics?
The zeroth law of thermodynamics is one of the four
laws of thermodynamics, which states that if two systems are in thermal
equilibrium with a third system, then they are in thermal equilibrium with one
another. As seen from the above section on thermal equilibrium, these three
objects will approach the same temperature.
Applications of the Zeroth Law of
Thermodynamics
The
zeroth law of thermodynamics is seen in many everyday situations.
- The thermometer may be the most well-known example of
the zeroth law in action. For example, say the thermostat in your bedroom
reads 67 degrees Fahrenheit. This means that the thermostat is in thermal
equilibrium with your bedroom. However, because of the zeroth law of the
thermodynamics, you can assume that both the room and other objects in the
room (say, a clock hanging in the wall) are also at 67 degrees Fahrenheit.
- Similar
to the above example, if you take a glass of ice water and a glass of hot
water and place them on the kitchen countertop for a few hours, they will
eventually reach thermal equilibrium with the room, with all 3 reaching
the same temperature.
- If you
place a package of meat in your freezer and leave it overnight, you assume
that the meat has reached the same temperature as the freezer and the
other items in the freezer